Samantha Cruz Rivera
University of Birmingham
Patient-reported outcomes (PROs) provide information about a patient’s health condition in terms of symptoms and quality of life from the patient perspective. PROs can inform healthcare and regulatory decisions, healthcare policy and can be used to provide timely care tailored to individual needs in routine clinical care.
The importance of PROs in clinical research is well known, but the ethical issues linked to it and how to address them are not. For example, the use of PROs may lead to uncertainty among patients about why data are being collected and used. In addition, reporting of PRO trial data is commonly inadequate, and PRO research frequently does not reflect the perspectives of underserved groups, failing to respect the participants’ autonomy. Therefore, we developed international guidelines that provide recommendations for ethical issues that should be addressed in PRO clinical research.
Since we developed the guidelines with the input of international collaborators— including patient partners—involved in PRO clinical research, we were not surprised by the different ethical issues highlighted. We would like to take this opportunity to thank ISOQOL members who were involved in the process.
The PRO ethics guidelines propose 14 key questions that capture core ethical issues (i.e., autonomy, justice, beneficence and non-maleficence). The key recommendations in the guidelines specify widely accepted ethical principles in the context of PROs and are framed in a way that is accessible to PRO researchers and useful for reviewers of PRO research. Included items focus on PRO-specific ethical issues relating to:
- research rationale
- objectives
- eligibility requirements
- PRO concepts/domains
- PRO assessment schedules
- sample size
- PRO data monitoring
- barriers to PRO completion
- participant acceptability and burden
- administration of PRO questionnaires for participants who are unable to self-report PRO data
- input on PRO strategy by patient partners or members of the public
- avoiding missing data and dissemination plans
Addressing ethical issues of PRO clinical research has the potential to ensure high-quality PRO data while minimising participant risk and burden, as well as to promote and protect participant autonomy and researchers and participants welfare. Furthermore, it may promote diverse, equitable and inclusive PRO research, the sharing of PRO research findings with participants and patients and minimisation of research waste.
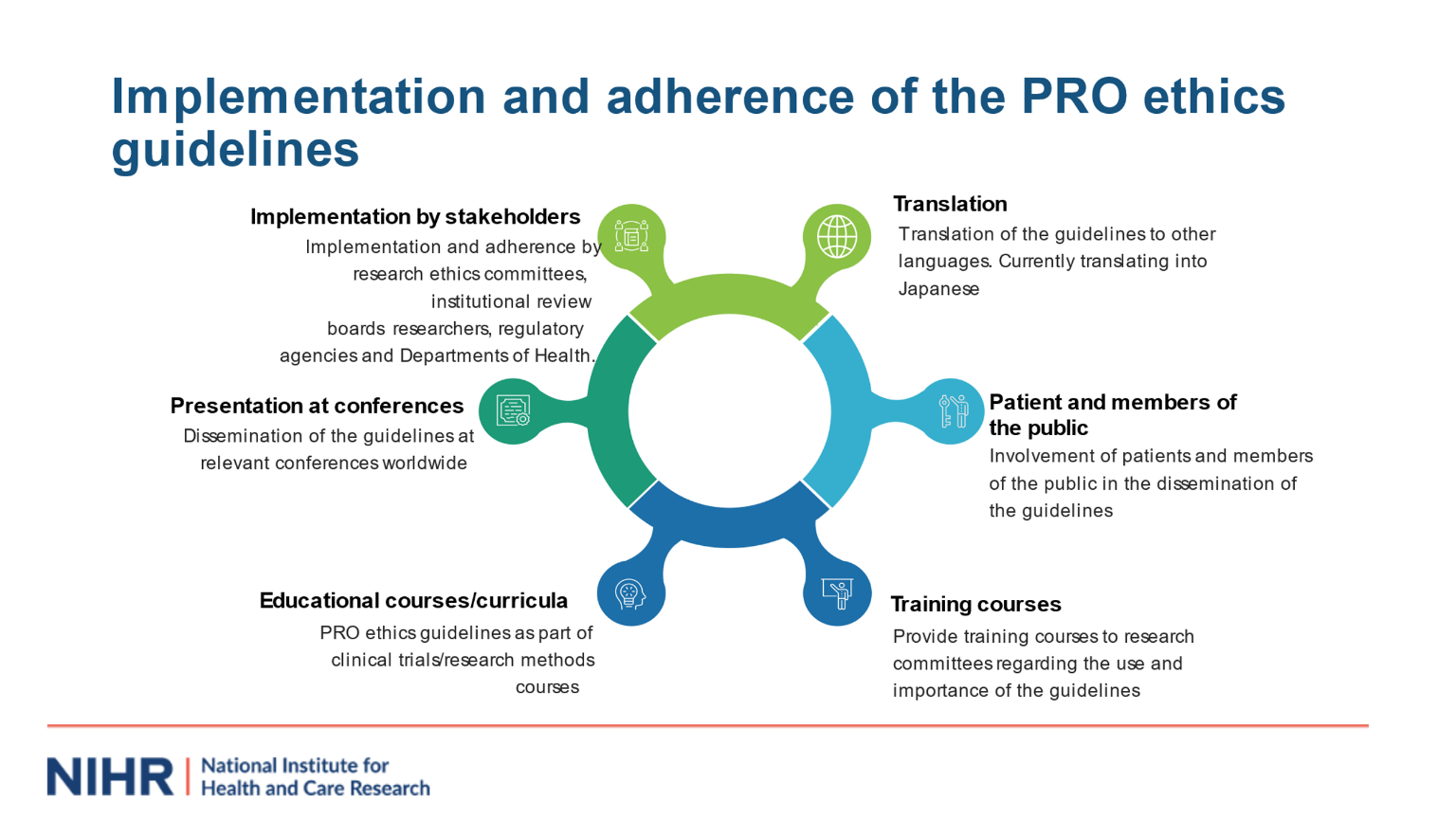
We hope the PRO ethics guidelines help identify ethical issues that should be considered by research groups, ethics committees, including patients, research participants and the public. We are planning further implementation of the PRO Ethics guidelines via the UK Health Research Authority, with collaborators from the Canadian Interagency Panel on Research Ethics and other international networks which will be described in future work. We would welcome your support in implementing the guidance, alongside other international guidelines and resources available at theproteusconsortium.org
Funding statement: This work was sponsored by the University of Birmingham, and funded by the NIHR Birmingham Biomedical Research Centre, UK Research and Innovation, UK SPINE, and the European Regional Development Fund.
Disclaimer: The views expressed in this article are those of the author(s) and not necessarily those of national agencies (e.g., the NIHR, FDA, Medicines and Healthcare products Regulatory Agency, Health Research Authority, Canadian Institutes of Healthcare Research, the Department of Health and Social Care, Canadian Interagency Panel on Research Ethics, or the Canadian Tri-Council Policy Statement).
Abstract will be presented in Oral Session 206 on Friday, 21 October, 3:40 pm – 5:10 pm.
This newsletter editorial represents the views of the author and does not necessarily reflect the views of ISOQOL.
How to Submit a Newsletter Editorial
Do you have something to share about health related quality of life and patient-centered outcomes? We want to hear from you!
Learn More

The International Society for Quality of Life Research (ISOQOL) is a global community of researchers, clinicians, health care professionals, industry professionals, consultants, and patient research partners advancing health related quality of life research (HRQL).
Together, we are creating a future in which patient perspective is integral to health research, care and policy.
